The Ultimate Guide to Silicone Breast Implants: Everything You Want To Know!15 min read

Phillip Chang
Board Certified Plastic Surgeon in Northern Virginia
Are silicone breast implants safe? The safety of silicone breast implants was studied by the Institute of Medicine in 2000. Based on their findings, the FDA allowed the re-introduction of silicone breast implants in 2006. They reported that silicone breast implants did not, with reasonable certainty, cause systemic health problems. Local complications such as implant rupture and capsular contracture, however, are potential risk factors.



What are breast implants?
Breast implants are implantable devices that are used to augment and enhance the fullness of a woman’s breast. They are filled with either saline (salt water) or silicone and come in various implant shapes and sizes.
What is not intuitive is that both saline implants and silicone-filled breast implants are made of a pliable silicone shell.
A short history about breast implants
Silicone implants surgery were largely banned in 1992 because the manufacturers could not at that time prove that they were safe and effective after health concerns arose.
At the time, a large number of lawsuits drove the main manufacturer, Dow Corning, into bankruptcy and led to payments worth billions of dollars to women who said they were harmed. Silicone breast implants were temporarily banned so that the FDA could further investigate their safety. That puts silicone implants’ age of legal incorporation into the breast augmentation market as fairly young.
Saline implants, however, remained on the market and remained the only implants available until 2006 when the moratorium on silicone implants was finally lifted.
Get Those Perfect Breasts
Paving the way, to the return of silicone implants was a large and comprehensive review by the Institute of Medicine in 2000.
The Institute of Medicine was a committee established by the U.S. government to review and present the the scientific information about silicone breast implants.
Are there problems with silicone filled breast implants?

Prior to 1992, silicone-filled breast implants were filled with liquid silicone. The concern was that there was a high rate of breast implant ruptures. It was unclear from the established data of the time whether the liquid silicone from a ruptured implant could result in multiple medical diseases including
- autoimmune diseases
- cancer
- capsular contracture
- neurologic disorders
- chronic pain disorders
- connective tissue disorders
Despite the lack of information either for or against any of these disorders, multiple class action lawsuits ensued even putting the largest manufacturer, Dow Corning, into bankruptcy.
Congress begins to study the safety of breast implants in 1997
In 1997, the U.S. House of Representatives asked the federal Department of Health and Human Services to sponsor an extensive study of silicone breast implants. This comprehensive evaluation would include the following:
- A scientific look at the components of implants, including an analysis of silicone chemistry, toxicology, and immunology
- Review the history of breast implants describe how implants have evolved
- Review the complications occurring during or after breast implant surgery
- Analysis and review whether silicone breast implants could be related to connective tissue, rheumatic, and neurological diseases and cancer
- Assess whether silicone implants could be detrimental pregnancy, breast-feeding, and children
- Evaluation of how mammography and other breast imaging techniques are affected by silicone implants
The Institute of Medicine: Part Of The National Academy of Sciences begins the investigation on the safety of breast implants
The Institute of Medicine was a 13-member committee that included 6 women that had distinguished themselves in medicine, science, and education; and who had experience in radiology, women’s health, neurology, oncology, silicone chemistry, rheumatology, immunology, epidemiology, internal medicine, and plastic surgery
An important task of the committee was to study and review thousands of published scientific reports. The committee also studied selected industry research reports on silicone breast implants and heard presentations from the public, including representatives of consumer groups, researchers, and women with silicone breast implants.
The goals of the committee were to produce recommendations regarding the need for further research on the safety of silicone breast implants and to provide information to women with breast implants or who were considering breast implant surgery.
Findings of the Institute of Medicine on the safety of breast implants
There is no evidence that Silicone Implants contribute to an increase in breast cancers. The committee felt that the evidence clearly shows that silicone breast implants do not cause breast cancer or the recurrence of breast cancer. In fact, some studies suggest that women with breast implants have fewer new or recurring cancers. See the FAQ below
There is no evidence that Silicone Implants contribute to an increase in autoimmune (connective tissue) diseases. These diseases cause the immune system which normally fights any invasion into the body, to produce antibodies that attack the body’s own tissues.Examples of autoimmune diseases are lupus, Raynaud’s phenomenon , rheumatoid arthritis, and scleroderma, a disease that involves thickening of the skin.
There is no evidence that Silicone Implants contribute to an increase in neurological diseases such as multiple sclerosis, neuritis, Lou Gehrig’s disease, or Ménière’s disease (a disorder of the inner ear) have been reported by some to be associated with breast implants, two large studies failed to find an increased incidence of these, or any other neurological diseases, in women with implants.
There is no evidence that Silicone Implants contribute to problems with breast feeding or with a developing fetus
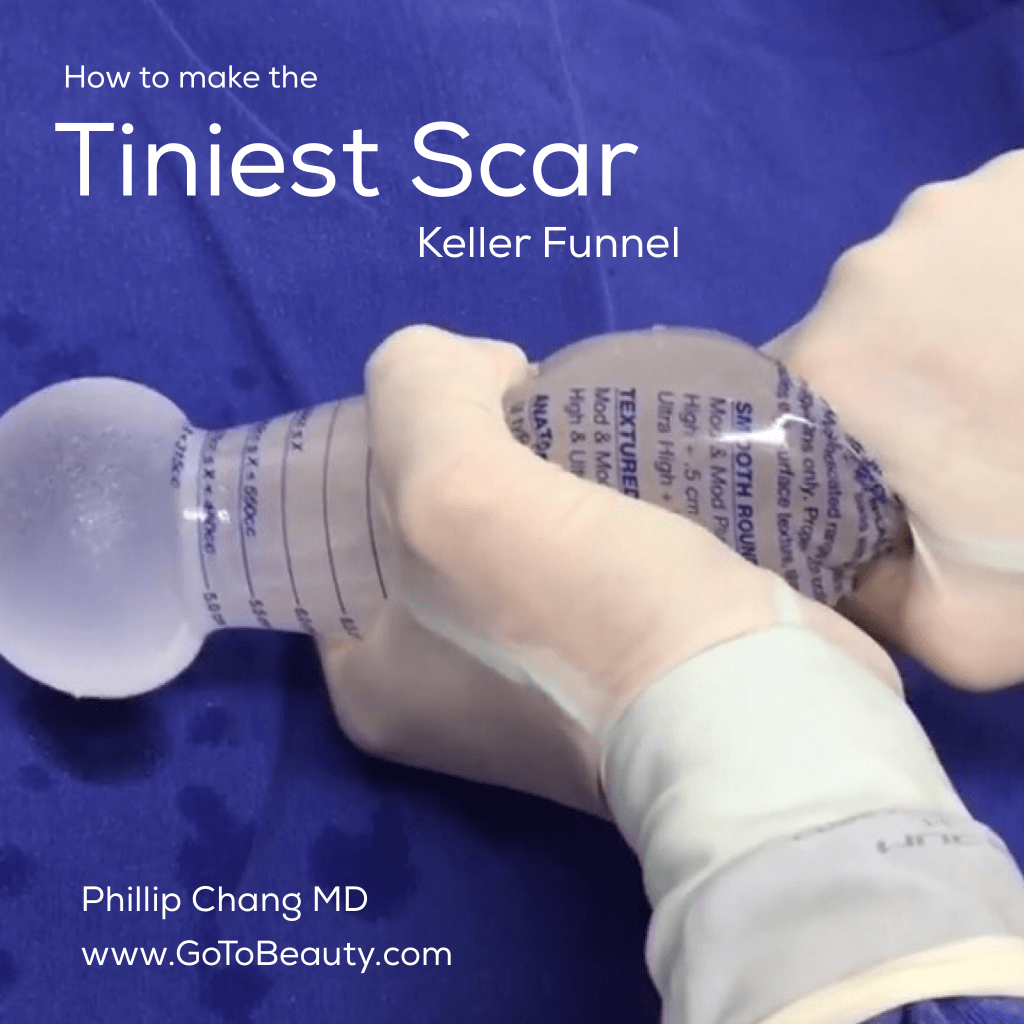
Keller Funnel is used to decrease bacterial contamination and trauma
Reported local complications after breast augmentation
Breast implant rupture, deflation, and capsular contracture are the main local complications that were cited by the IOM committee concerning silicone filled breast implants. Other potential complications, however include
- Infection
- Hematoma
- Pain
Infections occur in about 1-4 percent of breast augmentation procedures. The majority are local to the incision and do not present much of a problem. Generalized infections that involve the whole breast or the implant will most likely require the removal of the implant as the bacteria will colonize the foreign implant and will not be readily removed.
A hematoma is a blood collection within the breast pocket. Small hematomas will generally resolve on their own. Large ones are surgical emergencies that require drainage of the collection that can otherwise cause distortion of the breast or even skin and nipple death.
Subclinical infections can be thought of as bacterial contamination. Surgeons will due their due diligence in maintaining a sterile field during your surgery. This will include a surgical scrub, surgical gowns, sheets, and gloves. The system is, however, not foolproof. Bacterial live in the mild ducts for example. Contact with the bacteria might not be significant enough to cause an infection, but might be enough to create a “slime” layer around the implant which MIGHT be a cause of capsular contracture or chronic fatigue, muscle, or joint pain. These are only theories.
The committee report described one study of various implant devices, 93% of the women who reported pain also had an infection. When patients were given antibiotics (usually for staph) and implant devices were replaced by sterile models, 90% of the new implants were reported to be pain free.
FAQs about breast implant ruptures
- Tiny flaws in the shell
- Inadvertent needle pricks while the incision is being stitched up.
- Needle insertion (a biopsy, for example)
- If the breast is severely squeezed or compressed either during procedures to break up fibrous tissue (capsular contracture) around the implant
- Trauma caused by an automobile accident or even, some say, a too-tight hug.
- The implant shell may also weaken with time
An intracapsular implant rupture is a rupture of the implant that remains walled off from the rest of the breast and body by the fibrous capsule that normally forms around an implant. Because this type of rupture remains walled off from the body, this type of rupture may remain undetected
An extracapsular rupture is when the silicone rupture escapes both the implant shell and the biologic fibrous capsule around the implant. The silicone is able to escape the implant and enter the surrounding tissue.
It is not clear how frequently a silicone implant might rupture. The data on the subject has been very confusing and incomplete. This is because early implants did not require the manufacturer or plastic surgeons to report problems. The second reason is there were many manufacturers in the early history of silicone breast implants. There were no guidelines to how these implants were made at that time resulting in many varieties of implants with the potential for different safety profiles. Finally, ruptures are not always detected, the composition of implants has undergone many changes over the years, and the time interval varies and is not long enough to pick up late ruptures.
The investigators go as far as to say that no ruptures were found in late-model (“third-generation”) gel implants. They were, however, quick to say that more studies were needed.
The FDA maintains their recommendation that implants be replaced in 10 or 15 years
The IOM committee concluded that it is unclear whether implants in current use will need replacement in 10 or 15 years as some implants lasted longer than 15 years. The current recommendation is that implants should be replaced within this time-frame
FAQs about breast implant deflations
Breast implant deflation refers to the rupture of a saline breast implant. Deflation or leak of a saline breast implant is usually easy to see and diagnose as the implant is filled with water. The water will leak out of the implant similar to how water might leak out of a balloon. All or most of the water will leak out within a few hours to days.
The IOM committee concluded that deflation of modern first-year saline implants might run from 1 to 3% and that this percentage would rise steadily with time. The report strongly recommends that more studies be conducted to answer questions about today’s implant rupture and deflation rates.
The IOM committee concluded that deflation of modern first-year saline implants might run from 1 to 3% per year.
FAQs on breast augmentations and systemic concerns including cancer, breast feeding, and autoimmune diseases
There is reasonable certainty that silicone breast implants do not cause breast cancer. Two large studies have demonstrated that the the percentage of women who develop breast cancer after the placement of breast implants is actually slightly lower than the normal population.
The reasons have not been worked out but one explanation can be that the bodies immune system becomes hyper-vigilant in the presence of the foreign body. And that hyper-vigilance might cause the bodies early detection of some cancers
A large, 14-year study of 3,182 women with cosmetic implants actually showed fewer cases of cancer than would be expected in a group of that size.
There is no evidence to suggest that silicone breast implants cause autoimmune diseases, connective tissue diseases or rheumatic conditions. Importantly, the percent of women with autoimmune disease in the general population as the same as that reported in women with silicone breast implants. There is no evidence of cause and effect. The implication is that women with breast implants would likely have developed an autoimmune disease even if they did not get their implants.
The long-term Nurses’ Health Study involving 87,505 women showed no link between implants and connective tissue disease or rheumatic conditions. A review of 17 other studies also showed that there was no causal link with silicone breast implants.
The committee concluded that because there are more than 1.5 million American women with silicone breast implants, it would be expected that some of these women would develop connective tissue diseases, cancer, neurological diseases, or other systemic complaints or conditions during their life.
There is no evidence that breast implants affect the ability to breast feed.
There is no evidence of increased levels of disease or birth defects in children born to women with implants
Silicone implants do no appear to affect the immune system. Autoimmune disorders are diseases where your own immune system begins to attack your own body. Lupus and Sjogrens are examples of autoimmune disorders. Some critics have claimed that breast implants might provoke the body into producing antibodies against itself.
The Independent Review Group in the United Kingdom organized a study in response to women’s concerns about silicone breast implants; They concluded that silicone found in breast implants were bland substances with little toxicity and no adverse effect on the body’s immune system.
Silicone is a ubiquitous material that is used in a variety of foods, cosmetics, lubricants, and a variety of other consumer products. Silicone can even be found in baby’s formula.
Silicone can be found in the blood of patients who have never had silicone breast implants. This is presumably from eating or interacting with silicone just hanging out in the general environment.
The IOM committee concluded that silicon found in distant tissues to the breast most likely reflected human exposure to the widespread presence of silicon in the natural environment. The committee found that exposure of women to silicone from the breast implant is limited almost entirely to the implant, its capsule, and the tissue and lymph nodes immediately surrounding the area.
While systemic problems were not found with silicone breast implants, the committee reported that the most serious problems associated with the silicone breast implants were “local” complications.
Although generally not life threatening, such complications can cause discomfort and, in some cases, pose considerable risk. The IOM committee believes these local complications may prompt the need for additional medical procedures
The chances are great that most women will outlive their implants. The odds of needing to replace a breast implant over a lifetime is high.
A study of women receiving reconstruction over an average of 6 years showed that 16% of those with saline implants required replacements.
Another study reported an 18% implant loss in women reconstructed with gel implants or submuscular expanders.
A smaller study of women with implant troubles showed that, on average, over 12 years there were about three implants performed per woman.
- Rupture
- Deflation
- Capsular contracture
- Infections
- Hematomas ( local pooling of blood )
- Pain
- Implant displacement
Breast augmentation and capsular contracture
The body will under normal circumstances form a thin film or “capsule” around a breast implant. Therefore everyone will have a normal capsule around their breast implant. The human body considers a breast implant—or any implant—to be a foreign agent and forms a protective capsule of fibrous tissue around the intruder, resembling an immature scar.
The IOM Committee's Conclusions In 2000
The IOM committee’s review of research and medical studies shows a local, but not general, reaction to silicone breast implants.
- There is no evidence that silicone implants are responsible for any major diseases of the whole body. Women are exposed to silicone constantly in their daily lives.
- There is no plausible evidence of a novel autoimmune disease caused by implants.
- No increase in either primary or recurrent breast cancer in women with breast implants. Some studies even suggest lower rates of breast cancer in implanted women.
- No danger in breast-feeding;. In fact, cows’ milk and infant formulas have a far higher level of silicon than mothers’ milk.
- The major problems with implants are local, but not life-threatening, complications. These include implant removal, ruptures, deflations, capsular contracture, infection, and pain.
- Women may have secondary problems such as severe contracture, rupture, and implant removal.
- Implants do not last forever; risks accumulate over time, and many women should expect to have more than one implant.
- The committee suggests that women and their doctors consider submuscular implant placement, which makes diagnosis by mammography much easier and puts it more on a par with that available to women without implants.
The FDA lifts its ban on silicone breast implants in 2006
Partially based on the IOM committee findings and recommendations, silicone breast implants were re-introduced back into the market in 2006. They were able to demonstrate with reasonable certainty that silicone implants were reasonably safe and did not cause major systemic diseases including cancer, autoimmune diseases, immunologic diseases or neurologic problems
While silicone breast implants were not felt to cause systemic diseases, they do cause local problems including the potential for rupture, capsular contracture, infection, hematomas, and pain.
With the approval of the re-introduction of silicone breast implants, the FDA made the following recommendations
- It approved the devices for cosmetic breast augmentation in women age 22 and older and
- It approved the devices for breast reconstruction in women of all ages whose breasts have been disfigured or removed for medical reasons.
- MRI is recommended to check for silicone leaks every 2-3 years
- Replacement of the breast implants are recommended every 10 years.
Finding The Best Plastic Surgeon in Virginia

Dr. Phillip Chang is a leading plastic surgeon in Loudoun County who specializes in surgical and nonsurgical cosmetic procedures for the breast, body, face, and skin. He is board certified in plastic surgery by the American Board of Plastic Surgery and is the founder of Aesthetica Cosmetic Surgery & Laser Center in Leesburg, VA. Dr. Chang believes combining attentive care and minimally invasive techniques is the best avenue for achieving beautiful, natural-looking results.
Visit his office in Leesburg, Virginia in Loudoun County or fill out the contact form below for more information on how we can help you with your plastic surgery plans.
Let Us Help You!
Our office can provide you will helpful information, schedule a free consultation, and walk you through the process of having the procedure covered by your insurance.
Contact Dr. Chang's Office:
More Articles For You

The Latest Techniques in Double Chin Removal in Leesburg, VA
In the charming town of Leesburg, VA, where looking good and feeling great are top

The Art of Refining the Side Profile of a Woman through Plastic Surgery
In the realm of cosmetic enhancements, the side profile of a woman holds a pivotal

Enhance Now, Pay Later: Plastic Surgery Payment Plans in Leesburg, VA
In the picturesque town of Leesburg, VA, pursuing beauty and self-improvement is a journey many

Areola Reduction for Men in Loudoun County
In the heart of Loudoun County, where the beauty of nature meets bustling urban life,
